Naming Dynein Components and their Cytoplasmic Assembly Factors
HGNC, Guest Post ·This guest blog post has been contributed by Stephen M. King, one of our specialist advisors for nomenclature of dyneins and their cytoplasmic assembly factors.
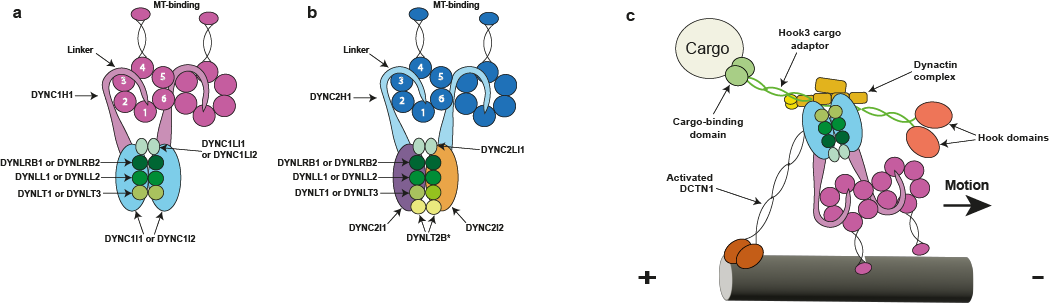
Figure 1 from Braschi et al., 2022 (a) The cytoplasmic dynein 1 complex. The DYNC1H1 protein heavy chains have large globular heads at the C-termini that are composed of a ring of six AAA+ domains. The microtubule-binding domains are located at the tips of antiparallel coiled coils that derive from AAA4. The linker/N-terminal domains connect the AAA rings and the intermediate and light chains. (b) The cytoplasmic dynein 2 complex. The DYNC2H1 protein heavy chains power retrograde IFT and have the same general domain organization as DYNC1H1. However, the tails of the two heavy chains fold differently due to an asymmetry imposed by the two different intermediate chains: one is straight while the other forms a zigzag shape and interacts with the IFT-B train (Toropova et al., 2019). The linker/N-terminal domain connects the AAA ring and the intermediate and light chains. *It remains unknown whether the DYNLT2B protein forms a homodimer or a heterodimer with another Tctex-type light chain. (c) Schematic showing the interaction between the dynein 1 and dynactin complexes. The adapter molecule affects the type of cargo bound; in this figure, the hook microtubule tethering protein 3 (HOOK3) encoded protein is acting as a cargo adapter.
C2021 Braschi et al. Originally published in the Journal of Cell Biology. https://doi.org/10.1083/jcb.202109014
Dyneins are multi-component motors that move along polarized microtubule tracks. These enzymes are found in most eukaryotic groups with a few notable exceptions such as angiosperm plants and red algae. Cytoplasmic dynein (Figure 1) is required for the movement of numerous cellular components such as nuclei and vesicles, and is even hijacked by invading viruses (think Herpes and HIV-1) to enable their transport from the cell periphery towards the nucleus.
Dyneins are also involved in building microtubule-based cellular extensions termed cilia that play key motile, sensory and secretory roles. A specialized dynein (Figure 1b) powers a process termed retrograde intraflagellar transport (IFT) that is needed to bring cilia assembly components, recycled materials and even activated signals from the ciliary tip back to the cell body.
The motility of cilia is driven by complex arrays of dyneins assembled along the ciliary axonemal microtubules (Figure 2); this motility can move individual cells such as sperm, whilst epithelia containing multiciliated cells generate fluid flow across surfaces, moving mucus in the lungs and cerebrospinal fluid in the ventricles of the brain.
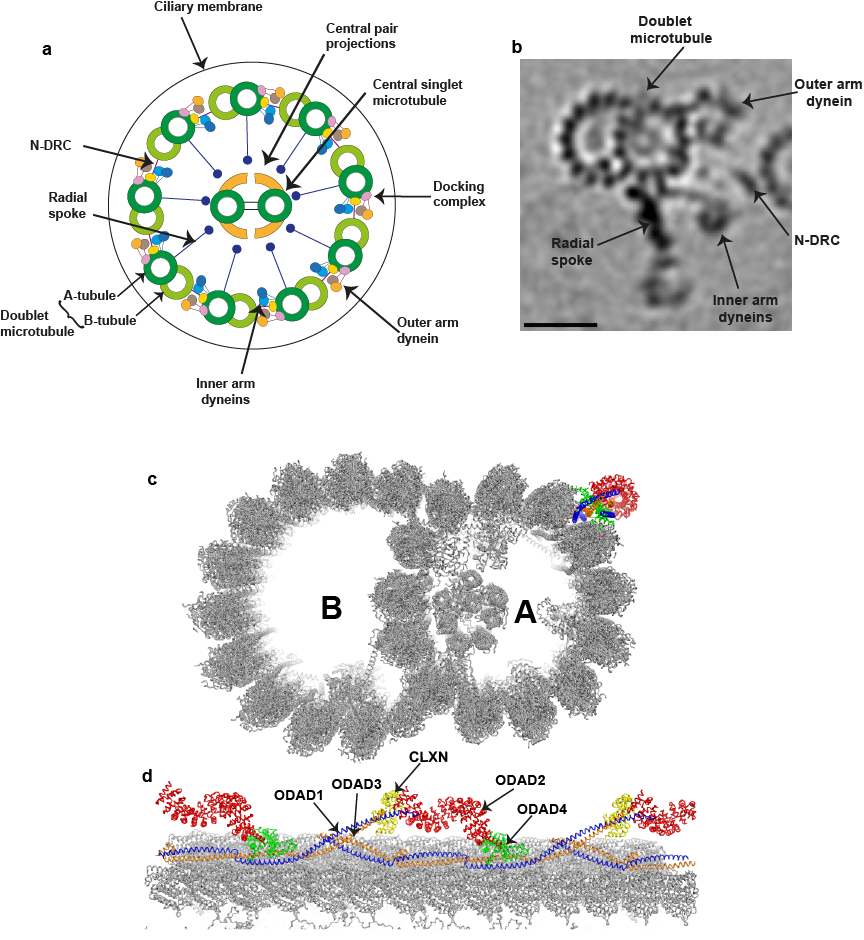
Figure 2, **Organization of a mammalian motile cilium. (from Braschi et al., 2022) a) The diagram illustrates the general 9+2 microtubule arrangement within the ciliary axoneme. The inner and outer rows of dynein arms generate the force required for ciliary beating. The N-DRC complex is a key regulatory structure that interconnects the doublet microtubules. The radial spokes regulate the beat of cilia by transducing signals between the doublets and the central microtubule pair. (b) Tomographic image of an averaged 96-nm repeat for a single human ciliary doublet microtubule, revealing the microtubule-associated dynein arms, N-DRC, and radial spoke. The scale bar represents 25 nm. This image was generated by Jason Schrad (Nicastro laboratory) using data from Lin et al. (2014). (c and d) Cross-section (c) and longitudinal (d) views of the 48-nm repeat organization of a bovine doublet microtubule. The components of the ODA-DC are individually colored and indicated. This ribbon diagram was generated with the PyMol molecular graphics system (Schrödinger) using Protein Data Bank accession no. 7RRO (Gui et al., 2021).
C2021 Braschi et al. Originally published in the Journal of Cell Biology.
https://doi.org/10.1083/jcb.202109014
Defects in dyneins lead to a broad array of phenotypes in humans. For example, mutations in a cytoplasmic dynein-associated component (encoded by PAFAH1B1 (platelet activating factor acetylhydrolase 1b regulatory subunit 1), also published using the alias “LIS1” (“lissencephaly-1”) lead to defects in brain development, while those in ciliary dyneins fall into two broad categories. Disruption of dynein-driven ciliary motility can cause male and female infertility, hydrocephalus, incorrect assignment of the left-right body axis leading to inverted placement of the internal organs, and is also associated with congenital heart disease. In contrast, alterations in the IFT dynein can affect cilia-based signaling, for example in the hedgehog pathway, and are especially associated with skeletal malformations.
Dyneins are built around large (~530 kDa) motor components that are associated with numerous other proteins required for assembly of these multi-motor complexes, binding them to appropriate cargoes and regulating their activity in order to generate useful work. For example, the axonemal outer dynein arm contains upwards of twenty different protein types. Dyneins have been studied for over 50 years and in that time many different dynein enzymes from a wide array of organisms (from green algae and sea urchin sperm, to ciliates and mammals) have been examined.
Even though many dynein components from these various organisms are closely related, this long history has led to a very confusing nomenclature. Previous efforts to clarify the naming schemes focused on cytoplasmic dynein and the closely related dynein that powers retrograde IFT (Pfister et al., 2005), and on categorizing the numerous dyneins involved in ciliary motility (Hom et al., 2011); this latter compilation also included several factors required in cytoplasm for assembly of ciliary dynein motors. Both these reports made major strides to reducing confusion in the literature. Since then numerous additional cytoplasmic factors have been identified that are needed for assembling dyneins, transporting them to cilia, and for controlling their activity prior to insertion into the axonemal superstructure.
Many of the human gene names associated with these factors were generic placeholders or based on some domain or motif but provided little functional information. This growing complexity has thus necessitated a new effort by (Braschi et al., 2022) to codify the nomenclature for human dynein-associated genes so as to make them more functionally relevant, and to reduce the potential confusion driven by the intricacy of dyneins themselves combined with their complicated cytoplasmic assembly.

This guest blog post was written by Stephen M. King, a professor of Molecular Biology and Biophysics and director of the Electron Microscopy Facility at the University of Connecticut Health Center. His research interests are in understanding the structure, function and regulation of dyneins especially those involved in the assembly and motility of cilia. His laboratory also studies the role of peptide amidation in ciliogenesis and the secretion of peptidergic signals in vesicles that bud from the ciliary membrane.
